Silicon, the second most abundant element in Earth’s crust, has been a cornerstone of human progress. Discovered in 1824 by Jöns Jakob Berzelius, it has journeyed from ancient flints to the heart of today’s technology. This element’s tale is etched in every smartphone screen and solar panel, with a global semiconductor market worth over $400 billion.
In this exploration of fun facts about silicon, we’ll delve into its profound impact on our lives, from the Silicon Valley revolutions to the silent strength it lends to skyscrapers. Join us as we uncover the versatile and vibrant chapters of silicon’s story.
1. Silicon: The Backbone of Technology
In the tech-savvy corners of the world, silicon reigns supreme. Discovered in 1824 by Jöns Jacob Berzelius, a Swedish chemist, silicon quickly became a symbol of innovation. Fast forward to the 1950s, and we see the first silicon transistors sparking a revolution. By the 1960s, the integrated circuit had paved the way for today’s computers, thanks to this versatile element.
Why is it the backbone, you might ask? Its ability to conduct electricity only under certain conditions makes it the perfect material for controlling electrical currents. Without silicon, there would be no microchips, no computers, no digital progress.
2. Silicon: Second Only to Oxygen
Silicon’s prevalence as the second most abundant element in the Earth’s crust was first quantified in the 18th century by Antoine Lavoisier, who identified it as a component of rock. This abundance is not just numerical; it’s functional. Silicon forms about 28% of the Earth’s crust by weight, a figure established through geological surveys. It is a key element that, together with oxygen, forms over 90% of the silicate minerals constituting the crust.
This omnipresence is why, throughout history, from the stone tools of prehistoric times to the glass in Roman palaces, silicon has been an unsung hero. The modern era appreciates silicon not just for its quantity but for its role in technology; without it, there would be no computers, no smartphones, and no digital revolution.
3. From Beach to Chip: Silicon’s Journey
The journey of silicon from a simple beach component to a complex chip is a story of transformation. Starting as simple silicon dioxide, through an intricate process involving temperatures over 1700°C, it becomes a pure, crystalline silicon ready to be sliced into wafers.
These wafers are then doped with other elements, meticulously crafted into intricate circuits. The first practical use of silicon in computing was in 1961 at Texas Instruments. Now, the global semiconductor silicon wafer market is projected to reach $12 billion by 2024, a testament to the element’s significance in the digital age.
Image: macitynet.it
4. Silicon’s Architectural Brilliance: Shaping Skylines
When architects dream, silicon helps bring those dreams to life. It’s the core of architectural marvels — from the shimmering glass of the Burj Khalifa to the robust concrete of the Hoover Dam. Introduced in the 19th century, structural silicone glazing now gives buildings their modern, sleek facades. Plus, silicon additives in concrete improve durability, a fact noted when the Pantheon in Rome, with its silica-rich Roman concrete, has stood the test of time for nearly two millennia.
The global market for construction glass was valued at approximately $121 billion in 2020, with predictions for growth, reflecting silicon’s ongoing importance in architecture.
5. Silicon in Agriculture: Boosting the Food Chain
Silicon’s role in agriculture is not just beneficial; it’s transformative. Studies suggest that silicon can increase drought resistance and reduce metal toxicity in plants, leading to healthier crops. While not traditionally considered an essential nutrient, its use in agriculture dates back to the 18th century when ‘silicate manures’ were first applied to European soils.
Recent research indicates that adding silicon to soil can boost the global rice yield by 5-10%, which is significant for food security. This represents millions of tons of additional food production, showcasing silicon’s potential impact on the global food chain.
Soo Ann Woon / Pexels
6. The Hefty Side of Silicon: Its Atomic Weight
With an atomic weight of 28.0855, silicon is anything but lightweight in the realm of science and industry. This atomic heft reflects its stability and reliability as a semiconductor material. It’s this very property that allows it to form stable and durable structures, both microscopically in chips and macroscopically in buildings.
The atomic weight of silicon also plays a crucial role in its ability to form crystals, which are vital in electronics and photonics. Understanding and manipulating these properties allows for advancements in computing power and efficiency, giving silicon its hefty reputation in technology.
7. Silicon’s Information Storage Capacity: The Data Vault
In the digital era, silicon is our information sentinel. The first silicon-based memory chip, the “Intel 1103”, introduced in 1970, paved the way for the memory storage revolution. Today, silicon-based flash memory is in nearly every portable electronic device. As of 2023, the global flash memory market size was valued at over $65 billion, demonstrating silicon’s pivotal role in data storage.
Silicon’s ability to store vast amounts of data in compact chips is a cornerstone of our digital lifestyle, from smartphones to the cloud. As data generation skyrockets, silicon’s role as the keeper of our digital existence becomes more critical.
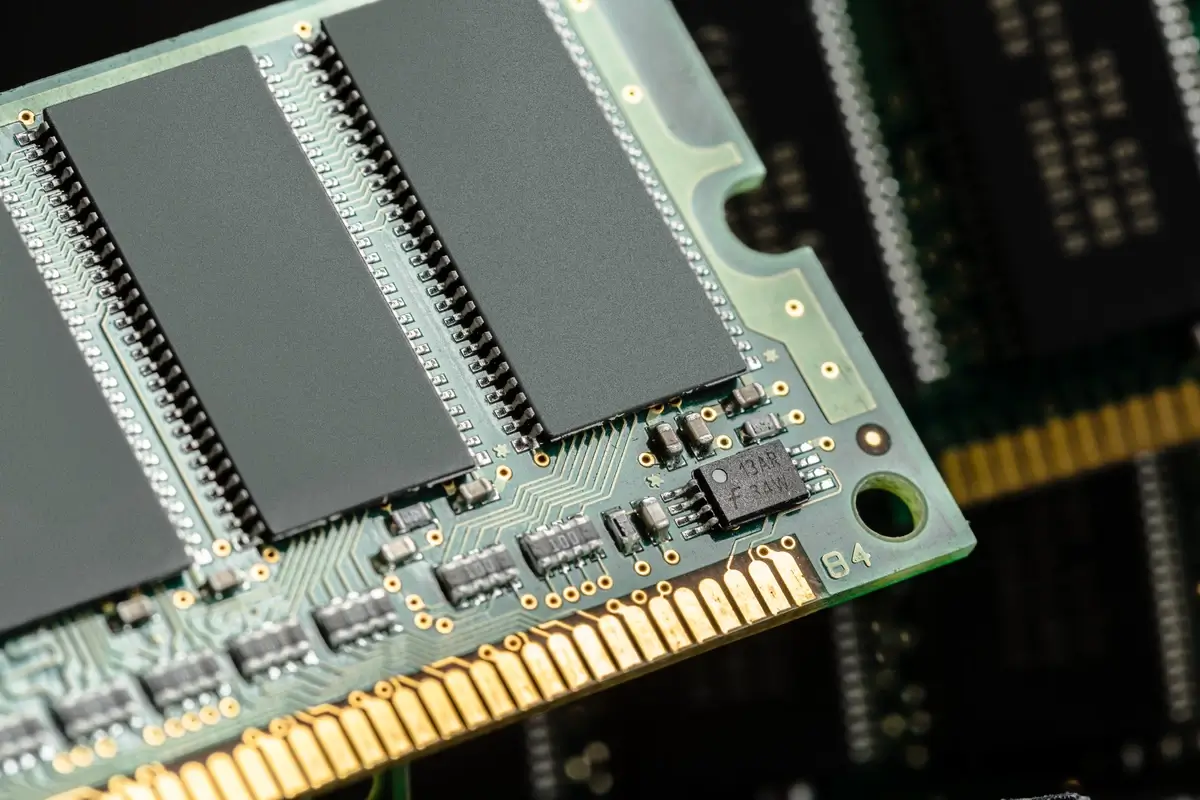
Image by fabrikasimf / Freepik
8. The Two Faces of Silicon: Solid yet Malleable
In its most common form, silicon is a hard, crystalline material—a testament to its solid state. Yet, this element, when highly purified, takes on a new persona in the tech world. Silicon can be manipulated at a molecular level, a quality that has been pivotal since the first silicon integrated circuits were developed in the late 1950s. This duality is what makes silicon essential in both construction (for its strength) and electronics (for its flexibility). For instance, the mechanical properties of silicon allow skyscrapers to withstand the stresses of wind and earthquakes, while its electrical properties are harnessed in processors to power our devices.
This transformative nature of silicon was first harnessed in 1954 with the development of the silicon solar cell by Bell Labs. Its ability to maintain structural integrity under high temperatures makes it invaluable to both architects and chip manufacturers.
9. The Silicon Lens: Revolutionizing Optics
Silicon lenses are capturing the world, one click at a time. The digital camera, debuting in the 1970s with silicon-based charge-coupled devices (CCDs), turned photography on its head. Now, with the rise of smartphones, billions of silicon-based image sensors are produced annually. The optical silicon market is expected to grow, reflecting its expanding role in our visually driven society.
These silicon wonders are not just in our pockets; they’re on Mars rovers, exploring the cosmos, and in medical devices, peering into the human body. Silicon’s clear advantage in optics is its precision and ability to be mass-produced, focusing our view of the universe.
Image: slrlounge.com
10. The Revolutionary Uses of Silicon
Silicon’s role in revolutionizing various industries can be traced back to the invention of the transistor in 1947 and the subsequent development of the silicon chip in the 1950s. This element’s ability to serve as a semiconductor revolutionized electronics, leading to the miniaturization and proliferation of devices. From the silicon photovoltaic cells that harness solar energy to the silicon-based aerogel that insulates space shuttles, the applications are groundbreaking.
Beyond electronics, silicon finds use in medical applications, such as in hydrogel form for contact lenses and as a lubricant in surgical tools. The diversity of silicon’s applications is a testament to its adaptability and its revolutionary impact on modern life.
11. Silicon Valley: More Than Just a Name
The term “Silicon Valley” was coined by journalist Don Hoefler in 1971, encapsulating the region’s emergence as a hub for innovation and the semiconductor industry. The valley’s name is a nod to the concentration of companies involved in the research and production of silicon-based semiconductor chips. The region’s growth was meteoric; by the 1980s, it had become synonymous with high-tech. Today, Silicon Valley is not just a geographic location; it’s a byword for the epicenter of technological advancement where companies continue to thrive on the silicon semiconductor legacy.
The valley’s economic impact is immense, with a GDP of over $275 billion, rivaling that of entire countries. Its influence on global technology trends and innovation ecosystems is unparalleled.
Image: sansaigonland.vn
12. Silicon: The High-Tech Element
Silicon’s reputation as the “high-tech element” stems from its central role in the development of integrated circuits and microprocessors. The first silicon microprocessor, the Intel 4004, was released in 1971, signaling the dawn of the personal computing era. Its properties as a semiconductor make it ideal for precision control of electrical currents in devices, which is essential for the computing industry.
As the technology evolved, silicon chips became more complex, now containing billions of transistors in a space smaller than a fingernail. The high-tech applications of silicon extend to telecommunications, automotive technology, and even quantum computing, making it an element that defines the cutting edge of technology.
13. Silicon’s Role in Renewable Energy
Silicon’s contribution to renewable energy is most prominently seen in the solar power industry. The first commercial silicon solar cell was made available in 1956, and since then, the efficiency of these cells has improved dramatically, with records surpassing 26% efficiency in laboratory settings. Silicon-based solar cells dominate the market due to their reliability and increasingly competitive cost.
The element’s role in renewable energy doesn’t stop at solar panels. Silicon is also used in wind turbines, battery technologies, and even in the development of more efficient electrical grids. As the world shifts towards sustainable energy, silicon’s role becomes ever more crucial, solidifying its position as a key player in the green revolution.
Image by senivpetro / Freepik
14. The Biocompatible Side of Silicon: Medical Marvels
Silicon isn’t just a building block of inanimate objects; it plays a significant role in biocompatibility in the medical field. Silicones, derivatives of silicon, are used extensively due to their flexibility, stability, and resistance to temperature and microbes. From artificial joints to tubing to drug delivery systems, silicones improve countless lives.
Beyond its structural uses, bioactive silicon compounds are also critical in various treatments, promoting tissue regeneration and bone growth. Silicon’s biocompatible side showcases its invaluable contributions to medical science and its role in advancing healthcare.
15. Silicon’s Invisible Hand in Art Preservation
While silicon’s technological uses are widely recognized, its contributions to art preservation are less known but equally significant. Silicon-based materials are used in the conservation of historical artifacts and buildings. These materials help protect against moisture and environmental degradation, ensuring that our cultural heritage withstands the test of time.
Silicones are also used in the restoration process of paintings and sculptures, providing a protective layer that is both inert and reversible, a crucial aspect in conservation. Thus, silicon’s invisible hand plays a pivotal role in safeguarding our artistic legacy for future generations.

Image: athensartconservation.com
FAQ
Are Silicon Rare?
Silicon is not rare; in fact, it is the second most abundant element in the Earth’s crust after oxygen, making up 27.7% of the crust by weight. Its abundance is evident in the vast expanses of sandy beaches, rocks, and clays found all over the planet.
Is Silicon Highly Flammable?
Silicon itself is not highly flammable. In its crystalline form, it is relatively inert and does not easily ignite. However, finely divided silicon powder can be flammable or explosive when exposed to air and can react with halogens or dilute alkalis.
What Are Two Advantages of Silicon?
Two significant advantages of silicon include its semiconductor properties, which make it essential for electronic devices, and its mechanical durability, which is utilized in the construction of buildings and the manufacturing of automobile parts. Its ability to conduct electricity selectively is fundamental to modern computing and telecommunications.
Who First Discovered Silicon?
Silicon was first discovered by the Swedish chemist Jöns Jakob Berzelius in 1824. He isolated silicon by heating chips of potassium in a silica container and then carefully washing away the byproducts.
How Did Silicon Get Its Name?
Silicon got its name from the Latin word ‘silex’ or ‘silicis,’ meaning flint. Berzelius named it “silicium,” from which the English word silicon is derived, referring to its common form as silica and in silicates.
What Are 5 Uses for Silicone?
Five common uses for silicone, a polymer derived from silicon, include:
- Sealants and adhesives in construction and manufacturing due to their durability and resistance to moisture.
- Medical devices and implants because of their biocompatibility and stability.
- Cooking utensils and bakeware as they are non-stick and can withstand high temperatures.
- Personal care products, such as conditioners and moisturizers, where they improve texture and moisture retention.
- Lubricants and greases for a wide range of mechanical and industrial applications because of their low chemical reactivity and high thermal stability.
Is Silicon a Metal or a Plastic?
Silicon is neither a metal nor a plastic; it is a metalloid, which means it has properties intermediate between metals and nonmetals. This allows it to conduct electricity under certain conditions, making it an ideal semiconductor.
What Are Chemical Properties of Silicon?
Chemically, silicon is a relatively inert element, but it can form compounds with 64 of the 96 stable elements. It is a semiconductor with an energy gap that allows it to conduct electricity at elevated temperatures. Silicon readily bonds with oxygen to form silicon dioxide, and it can also form silicon-carbide and various silicate minerals when combined with metals and other elements.