Did you know that there’s a whole day dedicated to celebrating an incredibly significant, albeit rather large, number in chemistry? Meet Mole Day, a unique celebration that intrigues both scientists and the curious-minded.
Though its name might make you think of a furry creature, it’s actually a day that revolves around 6.02 x 10^23, a number fundamental to chemistry. But why, you ask? Dive in to uncover the magic of this specialized holiday.
1. What in the World is Mole Day?
Delving into the annals of chemistry reveals the existence of Mole Day, a holiday celebrated with much fervor among those in the know. This isn’t about burrowing animals; instead, it’s a nod to Avogadro’s Number, a key measuring unit in chemistry. History traces this day back to the early 1980s when it was first observed, turning an abstract concept into a day of celebration.
For those who cherish the periodic table and chemical reactions, Mole Day holds a special place. It’s an opportunity to revel in the intricacies of chemistry and share its wonders with the world.
2. Celebration Time: October 23rd
Mole Day is celebrated on October 23rd, but there’s a whimsical reason behind this choice. The date mirrors Avogadro’s Number, 6.02 x 10^23, turning the time from 6:02 a.m. to 6:02 p.m. into a 12-hour chemistry fiesta. Historically, since its inception, the day has been marked with various academic and fun-filled activities across schools and colleges.
So, while the rest of the world may be indulged in regular autumn activities, for chemistry enthusiasts, this day is marked in bold, filled with mirth, mole puns, and molecular magic.
3. Avogadro’s Special Number: 6.02 x 10^23
When it comes to monumental figures in chemistry, 6.02 x 10^23, dubbed Avogadro’s Number, stands tall. This isn’t just any random number. Named after the Italian scientist Amedeo Avogadro, this figure represents the number of atoms in precisely 12 grams of carbon-12, a fact every chemistry student has etched in their memory.
The number, while colossal, plays a pivotal role. It’s the backbone for countless chemical calculations and reactions. Without it, our understanding of chemistry would be quite different, making its celebration all the more essential and intriguing.
4. Not About the Animal or Skin Mark!
In the English language, words often carry multiple meanings, and “mole” is a prime example. The word could refer to a small, burrowing mammal or a pigmented spot on the skin. However, in the realm of chemistry, a mole refers to Avogadro’s Number: 6.02 x 10^23. The concept of the mole was introduced in the early 20th century, and it’s a fundamental measuring unit that quantifies the amount of a substance.
The term “mole” in chemistry is derived from “molekulargewicht,” a German word meaning molecular weight. This chemical mole might not dig in the dirt or decorate your skin, but it sure digs deep into the realm of atoms and molecules.
5. It’s a Chemist’s Dream Holiday
While Valentine’s Day celebrates love and Halloween celebrates spookiness, Mole Day celebrates a love for science. The interest in this day peaked in the 1980s when schools started adopting the celebration, highlighting the basic principles of chemistry. A particular highlight is the Mole Day theme, which changes every year. For instance, in 2019, the theme was “Mole-o-Ween.”
It’s not just about fun; the day is rooted in education. For instance, in 2017, a study highlighted how Mole Day activities enhanced student understanding of the otherwise abstract mole concept, bridging theory with tangible learning.
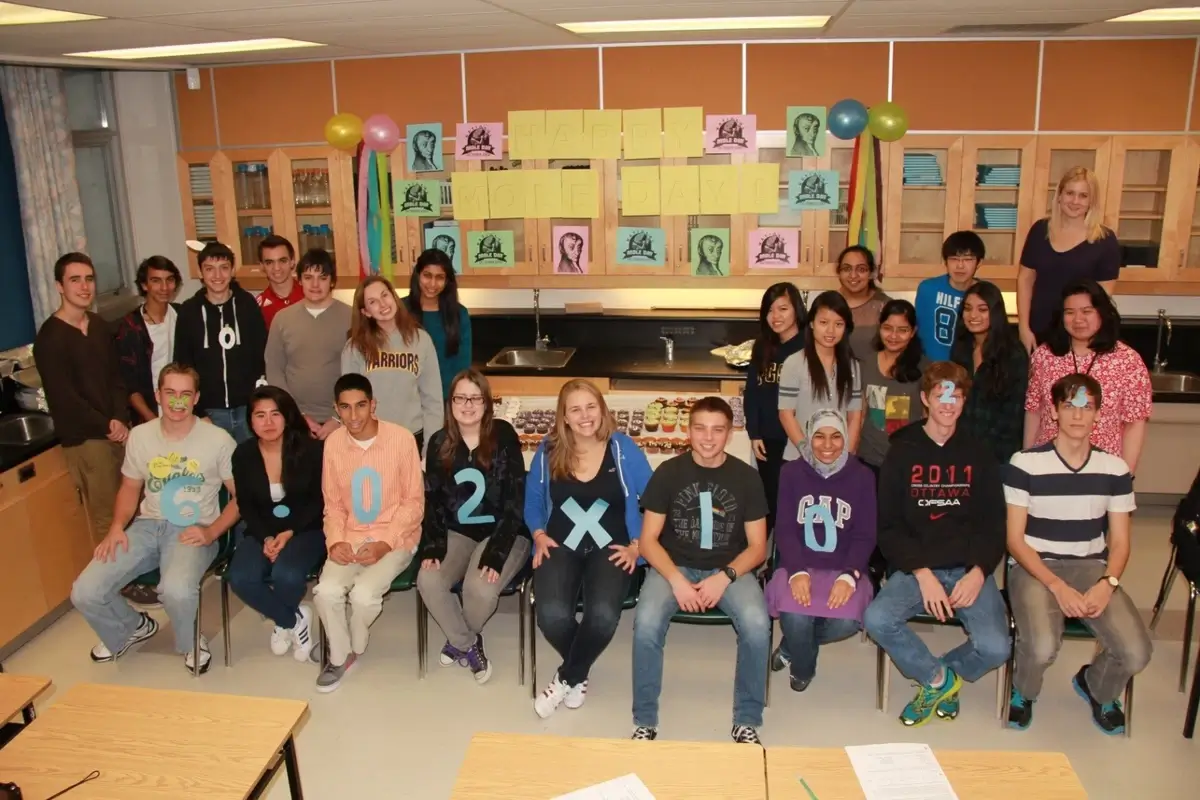
Image: uwaterloo.ca
6. National Chemistry Week’s Shining Moment
National Chemistry Week, established in 1987 by the American Chemical Society, is an annual event promoting the wonders of chemistry. Its goal? To reach out to the general public, especially students, to spark an interest in the world of molecules and reactions. Mole Day, celebrated on October 23rd, is the standout event of the week.
Data from ACS shows that over the years, schools participating in National Chemistry Week witnessed an increased interest in chemistry as a subject, thanks to fun-filled, yet educational events like Mole Day.
7. Mole Day Around the World
From its American roots, Mole Day has burrowed its way across continents. In Canada, for instance, the Toronto District School Board reported in 2018 a surge in Mole Day participation. Meanwhile, in the UK, institutions like the Royal Society of Chemistry have been instrumental in spreading the Mole Day word, often tying it with their outreach programs.
In Australia, universities have integrated Mole Day into their curriculum, using it as a platform to merge fun with foundational chemical learning. It’s evident: Mole Day’s appeal is universal, resonating with anyone keen on understanding the world at its most basic, molecular level.
8. Mole-themed Desserts and Treats
Mole Day isn’t just a celebration of numbers and atoms – it’s also an occasion that tickles the taste buds! Around the globe, schools and institutions use the occasion as an excuse to whip up mole-themed treats. Brownies, for instance, are often dubbed “mole hills,” while gummy worms represent burrowing moles. A report by the American Chemical Society in 2016 even detailed a recipe competition centered around Mole Day, with participants bringing in dishes like “Mole-asses cookies.”
This tasty approach to the day not only satiates hunger but also offers a delightful, edible way to make abstract concepts more digestible.
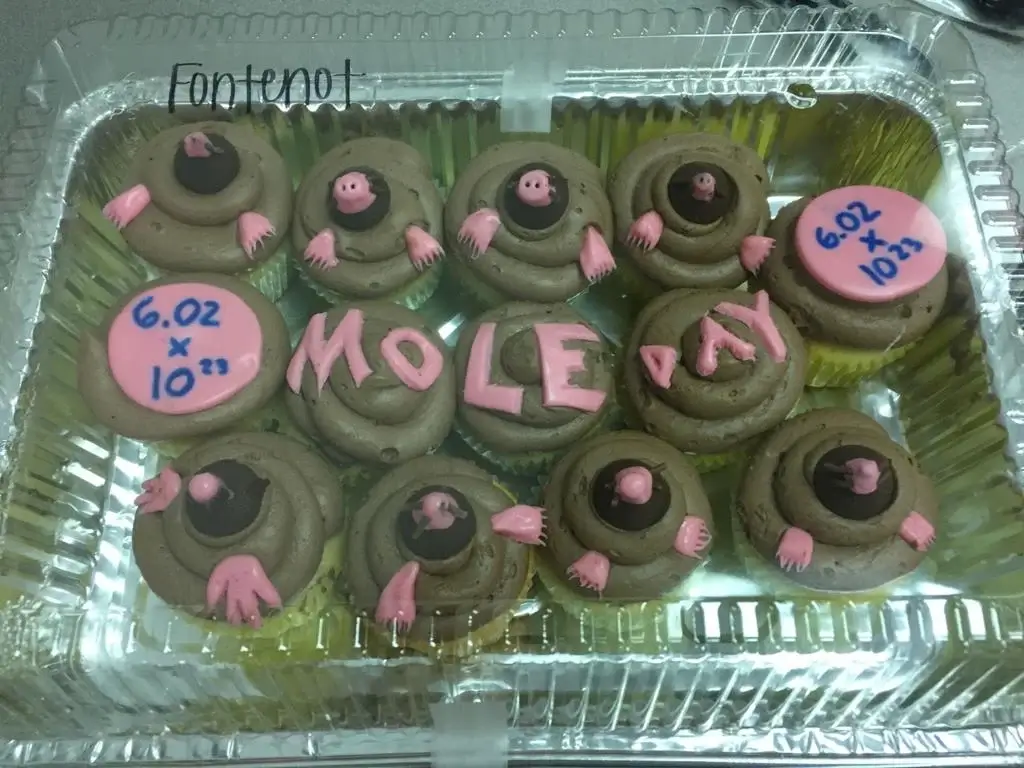
Image: theadvocate.com
9. Atoms, Molecules, and Avogadro!
The mole concept hinges on the understanding of atoms and molecules. One mole of any substance contains 6.02 x 10^23 atoms or molecules. But why this specific number? This fundamental concept, introduced by Amedeo Avogadro, is often taught in high schools and universities as Avogadro’s constant. This number isn’t arbitrary. It’s derived from the number of atoms found in 12 grams of carbon-12.
Surprisingly, this concept isn’t as old as one might think. It was only in 1971 that the 14th General Conference on Weights and Measures formally defined the mole, grounding it in Avogadro’s pioneering work.
10. Amedeo’s Legacy Beyond Moles
While Avogadro is most famously linked to the mole concept, his legacy in chemistry and physics extends much further. Born in 1776 in Turin, Italy, Amedeo Avogadro was not just a chemist but also a physicist. His contributions to molecular theory and his hypothesis that equal volumes of gases contain equal numbers of molecules were groundbreaking.
In honor of his immense contributions, in 1954, the International Union of Pure and Applied Chemistry decided to name the number 6.02 x 10^23 as Avogadro’s number, cementing his legacy in the annals of scientific history.
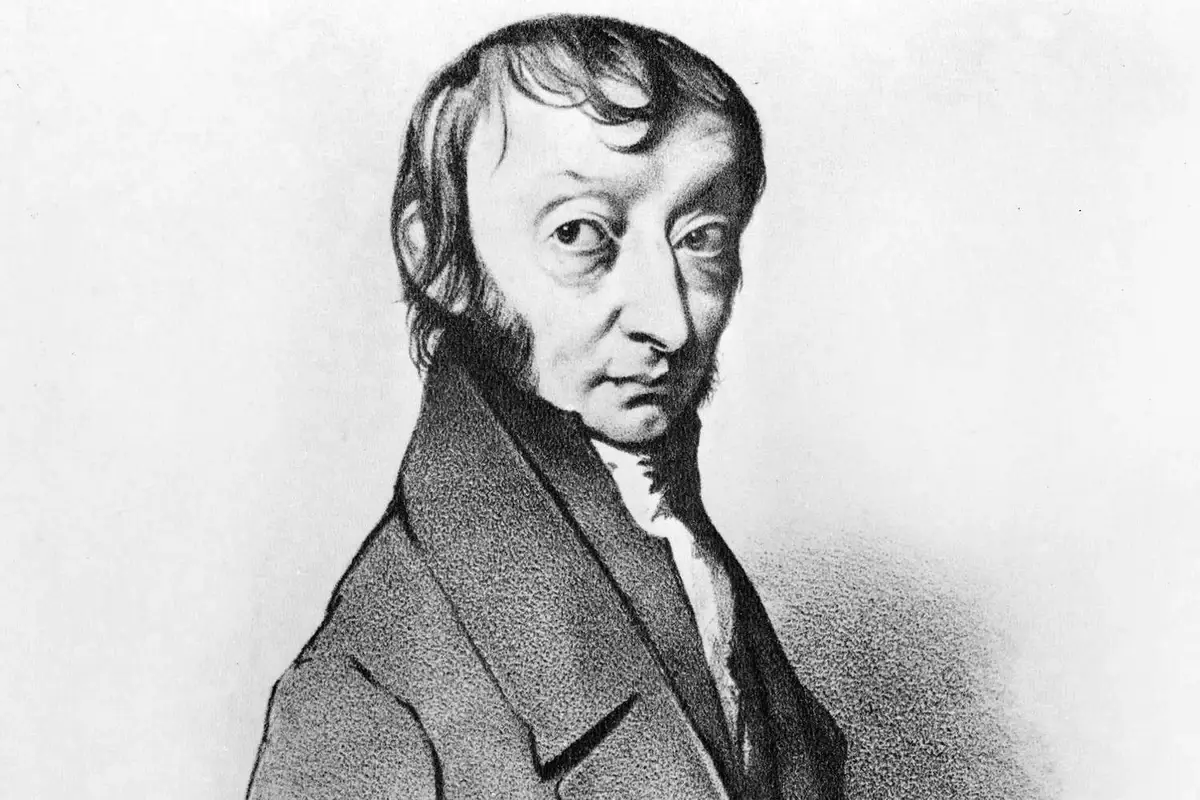
Image: thoughtco.com
11. The Basic Unit of Chemistry Measurement
The concept of the mole is deeply ingrained in the world of chemistry, acting as the basic unit for measuring the amount of a substance. Imagine trying to count the grains of sand on a beach or the stars in the sky – daunting, right? The mole provides a practical method of measurement, making those vast quantities manageable. In terms of real-world applications, the mole concept is pivotal in drug development, manufacturing processes, and even in space research.
Without the mole, many calculations in chemistry would be unfeasibly complicated. Its inception has simplified complex concepts, making them accessible to learners and professionals alike.
12. How the Mole Influences Our Daily Lives
While the mole might seem like a lofty concept, reserved for the hallowed halls of academia, it impacts our daily lives in more ways than we realize. From the pharmaceuticals we consume, determined by precise molar concentrations, to the fuel that powers our cars, calculated through mole-based reactions, this unit is omnipresent. For instance, water treatment plants utilize molar calculations to ensure the right amount of chlorine is added to disinfect water, making it safe to drink.
Thus, while the term “mole” might draw blank stares from many, it silently plays a pivotal role in our day-to-day lives, ensuring precision in myriad processes.
13. Mole Day Merchandise and Memorabilia
For those truly smitten by Mole Day, there’s a world of merchandise and memorabilia waiting to be explored! From T-shirts emblazoned with witty mole puns to intricately designed mole jewelry, there’s no shortage of items to showcase one’s love for this chemical holiday. The National Mole Day Foundation even has a catalog that features an array of mole-centric items, including notebooks, pins, and posters.
These pieces of merchandise not only serve as tokens of appreciation for the mole concept but also foster a sense of community among chemistry enthusiasts worldwide.
14. Fun Fact: Avogadro Wasn’t the First to Define the Mole
A shocker for many, but Amedeo Avogadro, while instrumental in refining and promoting the mole concept, wasn’t its sole pioneer. The roots of the concept can be traced back to Jean Baptiste Perrin, a French physicist. In the early 20th century, Perrin received the Nobel Prize for his work on the discontinuous structure of matter, which included determinations of Avogadro’s number.
It was only after Perrin’s findings that the term ‘mole’ was more firmly associated with Avogadro, honoring his foundational work in molecular theory.
15. Innovative Ways Schools Celebrate
Schools are at the forefront of Mole Day celebrations, and their innovative approaches to the day are nothing short of inspiring. In a survey conducted by the American Chemical Society in 2020, it was found that schools employ methods ranging from mole-themed escape rooms, mole rap battles, to mole scavenger hunts. In the digital age, some schools have even introduced virtual reality experiences, helping students visualize Avogadro’s number in interactive environments.
Such innovative celebrations not only make learning engaging but also pave the way for a more hands-on, immersive educational experience.
FAQ
When is mole day in chemistry celebrated and why?
Mole Day in chemistry is celebrated annually on October 23rd, from 6:02 a.m. to 6:02 p.m. Why such a specific time frame? Well, it’s all about the number: 6.02 x 10^23. This number, known as Avogadro’s number, represents the number of atoms or molecules in one mole of any substance. The date and time playfully mimic this number, bringing attention to this foundational concept in chemistry. The idea behind celebrating this day is to honor Amedeo Avogadro, the Italian scientist who significantly contributed to molecular theory and whose name the number carries.
What is interesting about Mole Day?
Mole Day is not just another academic observance; it’s a quirky fusion of science, creativity, and humor. While the concept of the mole and Avogadro’s number are undeniably academic, the way the day is celebrated brings a lighter, more entertaining side to chemistry. The day often features puns, jokes, and even mole-themed songs and dances. It bridges the gap between hardcore science and popular culture, making chemistry more accessible and relatable. Moreover, it’s not often that a numerical constant gets its own global recognition day, making Mole Day truly unique in the annals of science celebrations.
How is mole day celebrated?
Mole Day is celebrated with a mix of educational activities and light-hearted fun. Schools and universities, in particular, often organize events that aim to educate students about the importance of the mole in chemistry through interactive activities. Common celebrations include baking mole-themed desserts (like “guacamole” and “mole-asses” cookies), wearing mole-related attire, and even participating in mole-themed quiz competitions. Some institutions also host lectures, workshops, and demonstrations that dive deep into the concept of the mole. Additionally, communities around the globe come together, both offline and online, sharing mole memes, jokes, and even composing mole-related music and poetry, showcasing the fun side of chemistry.